
Fucoidan and Its Health Benefits
Peili Shen, Zongmei Yin, Guiyan Qu, Chunxia Wang
QINGDAO BRIGHTMOON SEAWEED GROUP, QINGDAO, CHINA
Introduction
Fucoidan designates a group of certain fucose-containing sulfated polysaccharides that not only have a backbone built of (1 → 3)-linked α-l-fucopyranosyl or of alternating (1 → 3)- and (1 → 4)-linked α-l-fucopyranosyl residues but also include sulfated galactofucans with backbones built of (1 → 6)-β-d-galacto- and/or (1 → 2)-β-d-mannopyranosyl units with fucose or fuco-oligosaccharide branching and/or glucuronic acid, xylose, or glu- cose substitutions (Ale et al., 2011). Fucoidan was first discovered by Kylin in 1913. It is a water-soluble natural sulfated polysaccharide existing mainly in the cell wall and mucous matrix of various species of brown seaweeds, such as mozuku, kombu, limu moui, blad- derwrack, and wakame (Atashrazm et al., 2015), accounting for about 0.3%–1.5% of the wet weight of brown seaweeds. Various forms of fucoidan have also been found in some marine invertebrates such as echinoderms (Mulloy et al., 1994). Owing to its many bioac- tivities, fucoidan is now widely used in functional foods, pharmaceuticals, cosmetics, and many other health-related products, using its anticoagulant, antitumor, antithrombotic, antiviral, antioxidation, and antirenal failure properties as well as immunomodulation, enzyme inhibition, and many other unique bioactivities (Xue et al., 2001).
This chapter intends to offer a review of the manufacturing process for fucoidan and summarize its chemical structure, bioactivities, and applications in the functional food industry.
Extraction of Fucoidan From Brown Seaweeds
1. Crude Extraction
There are many methods for the extraction of fucoidan from brown seaweeds, using spe- cies of Chordariales, Laminariales, Ectocarpales, and Fucales as the main raw materi- als (Mabeau et al., 1990). The extraction methods involve a simple dissolution in water, dilute acidic or alkaline extraction, solvent extraction, enzyme-based extraction, micro- wave-assisted extraction, and ultrasonic-assisted extraction, with effective extraction often involving the combination of two or more of these methods. In the early stages of its development, the extraction procedures used soaking in aqueous Na2CO3 solution as the first step to break the seaweed biomass; however, this treatment converts alginic acid to its sodium salt, resulting in alginate and other sugar impurities in fucoidan. Water or slightly acidic solutions are now commonly used to extract fucoidan from the seaweed biomass.
New extraction techniques have been applied to the production of fucoidan from brown seaweeds. For example, microwave-assisted extraction technique has a high yield and a low extraction time, even though its effect on the structure and bioactivities of fucoidan is still unknown. Ultrasound-assisted extraction can be used to disrupt the cell wall, although it may result in the degradation of fucoidan. For these two methods, it is recommended that the extraction condition is mild to avoid any structural change to fucoidan. Enzyme-assisted extraction of fucoidan is performed under moderate condi- tions, where the enzymes used include alginate lyase and laminarinase, which do not hydrolyze fucoidan and at the same time, liberate it through the degradation of the sea- weed cell structures.
Overall, the extraction of fucoidan retaining its original molecular weight, sulfate ester content, and sulfating position requires several time-consuming and expensive chromato- graphic or fractionation steps. As shown in Fig. 11.1, the extraction of fucoidan is typically performed by soaking the raw seaweeds in aqueous or acidic solutions at temperatures
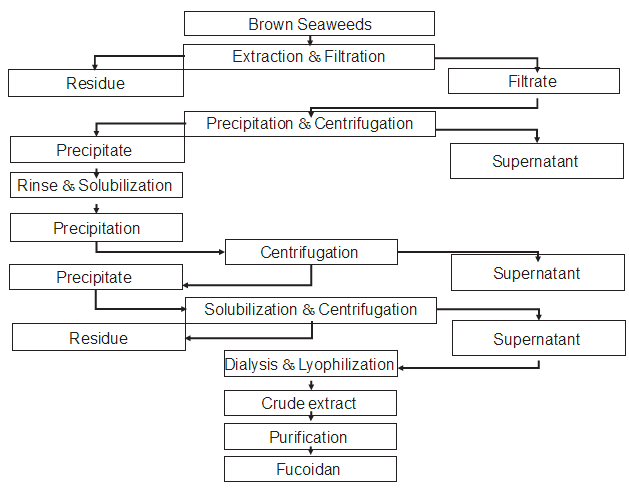
FIGURE 11.1 Schematic illustration of the extraction of fucoidan from brown seaweeds.
ranging from 70–100°C for several hours, followed by ethanol precipitation where salts and small molecules are removed from the fucoidan fractions. The precipitates are then washed, dissolved, and treated with CaCl2 or quaternary ammonium salt solution (cetyl- trimethylammonium bromide, CTAB) to form precipitates again, which are then dissolved and dialyzed before being lyophilized to obtain the crude extract. Extraction conditions such as solvent polarity, pH, temperature, and time are closely related to the structure and purity of the fucoidan. The available structural data evidently indicate that the variation in the composition of fucoidan extracted from different methods affects bioactivities (Yang et al., 2008).
2. Purification
The crude extract of fucoidan generally contains sodium alginate, protein, pigment, inorganic salt, and other impurities, which adversely affect the color, flavor, and bioac- tivities of the product. Purified fucoidan can be obtained by column chromatography, mainly gel filtration chromatography and ion-exchange chromatography. The traditional method of ethanol precipitation is used for desalting and removing low-molecular weight compounds, whereas gel filtration chromatography is mainly used to separate fucoidan with different molecular weights. Ion-exchange chromatography is based on the differ- ent charges of polysaccharides, such as sepharose CL6B, sephadex G-50, DEAE-cellulose, DEAE-toyopearl 650M, and sephacryl S-400. The desalting and separation from other compounds can be performed simultaneously. For example, chromatographic decolor- ation using a hydrophobic carrier material can be used to separate hydrophobic substances such as flavins and polyphenols from fucoidan. Although anion-exchange chromatogra- phy is complicated and time-consuming, it is a powerful tool to purify fucoidan because of the sulfate groups in its structure.
A novel fast purification tool has been developed for recovering fucoidan from Fucus vesiculosus by dye affinity chromatography based on amino-derivatized Sepabeads EC-EA. The purity is improved by 1.46-fold, with a yield of 7.5% from dried seaweed powder (Hahn et al., 2016).
Chemical and Physical Characteristics of Fucoidan
Fucoidan is soluble in water but insoluble in ethanol, acetone, chloroform, and other organic solvents. The pH of its aqueous solution is 6.46, which is slightly acidic. Chemically, fucoidan is a polysaccharide with complex structures. Although its monomers are mainly l-fucose with sulfate groups on the sugar chain, both the l-fucose and sulfate contents vary significantly for fucoidan extracted from different species of brown seaweeds and under different processing conditions. Fucoidan prepared from F. vesiculosus is composed of 44.1% fucose, 26.3% sulfate, and 31.1% ash (Black et al., 1953; Takashi et al., 1994). The typical l-fucose content is 30%–45%.
Fig. 11.2 shows the typical molecular conformation of fucoidan (Cumashi et al., 2007; Ale and Meyer, 2013), where the fucose monomers are present in a linear backbone built up of (1 → 3) linkage or alternating (1 → 3) and (1 → 4) linkages, with (1 → 2) linked branches also present in the chemical structure of fucoidan. Sulfate groups are mainly substituted at
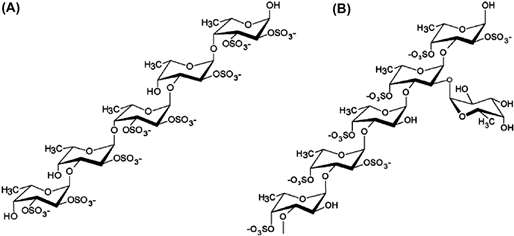
FIGURE 11.2 Typical structure of fucoidan from different brown seaweeds. (A) The fucose monomers are present in a linear backbone built up of alternating (1 → 3) and (1 → 4) linkages, with sulfate groups substituted at the C2 or C3 position. (B) The linear backbone is mainly built up of (1 → 3) linkage, with (1 → 2) linked branches occasionally. Sulfate groups are substituted at the C4 or C2.
the C2 or C4 and occasionally at C3 position on the fucose residues (Silchenko et al., 2013; Conchie and Percival, 1950; Manish et al., 1993; Tissot et al., 2006). On average, each fucose residue contains 1–2 sulfate groups. The fucose groups are also attached to the main poly- meric chain to form branched side chains, which further complicates the complex struc- ture of fucoidan extracted from many different seaweed species (Bilan et al., 2002, 2004; Lionel et al., 2001; Marais and Joseleau, 2001).
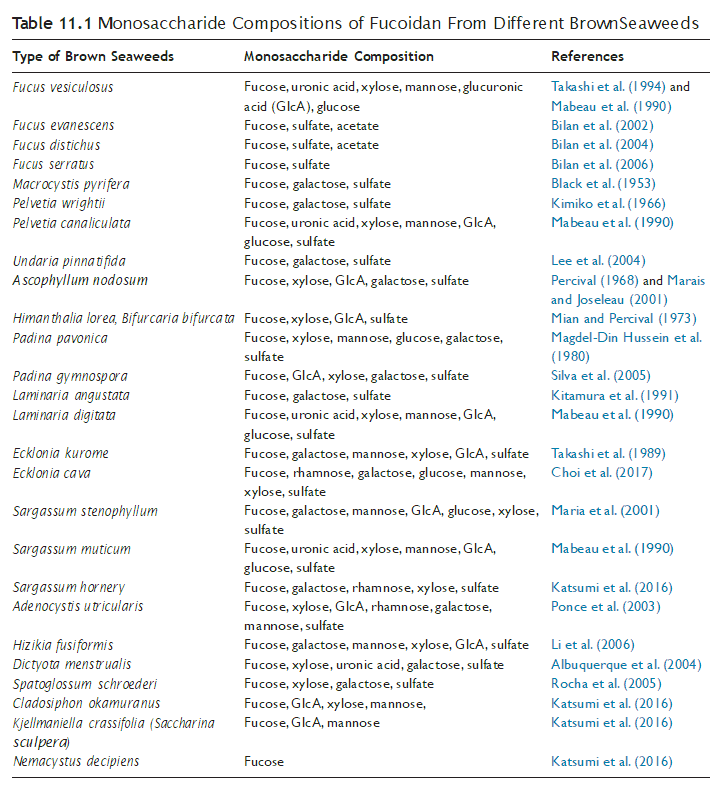
It should be pointed out that fucoidan is not a pure fucan sulfate. It is a heteropolymer containing various amounts of other monosaccharides such as galactose, xylose, man- nose, and uronic acids distributed on the sugar chain irregularly (Mabeau et al., 1990; Bilan et al., 2006). It has been reported that the other monosaccharides in the fucoidan structure are in a highly heterogeneous and branched structure. They are not sulfated and are pres- ent on the periphery of highly branched molecules (Mian and Percival, 1973; Magdel-Din Hussein et al., 1980; Lionel et al., 2001). Table 11.1 summarizes the monosaccharide com- positions of fucoidan from different brown seaweeds.
The fucoidan structure and monosaccharide composition are affected by several factors, including the species of seaweeds, the time and location of harvesting, and the extraction method and conditions (Black, 1954). At the same time, the physiological activ- ities of fucoidan are closely related to its complex structure such as molecular weight, monosaccharide composition, sulfate content and position, linkage mode, and sequence of monosaccharide residues. Many studies have been carried out to assess the relation- ship between the chemical structure and bioactivities of fucoidan. In particular, the sulfate content of fucoidan is attracting a lot of research efforts. Because sulfation is a key factor in improving fucoidan bioactivity, there have been many attempts to produce oversul- fated fucoidans to enhance its biological properties (Ferial et al., 2000). Cho et al. (2010) studied the effect of oversulfation on the in vitro anticancer activity of fucoidan and found that significant differences in anticancer activities were observed after the addition of sulfate groups. Results suggested that oversulfation causes higher negative charge in the molecule that can facilitate the formation of fucoidan–protein complexes involved in cell proliferation, where the polyanionic nature of fucoidan is necessary for antiviral proper- ties. It was shown that the modified galactofucan had a strong inhibitory effect on herpes simplex virus 1 and 2, whereas uronofucoidan prepared by extraction at 70°C had no anti- viral activity (Ponce et al., 2003). Galactofucan is mainly composed of fucose, galactose, and sulfate, whereas uronofucoidan is mainly composed of fucose and uronic acid, with very low sulfate content. The sulfate groups in fucoidan appear to have a significant effect on its antiviral activity, which is similar to results shown for other sulfated polysaccharides and monosaccharides (Bagasra et al., 1991; McCulure et al., 1991).
In addition to sulfation, the degree of branching also has a significant effect on the
bioactivities of fucoidan, with higher degrees of branching corresponding to more signifi- cant cytotoxicity in antitumor effects of fucoidan (Catarina et al., 2017). Molecular weight is another crucial factor for fucoidan as a bioactive agent. Cho et al. (2010) reported that among the different fractions of fucoidan, the F5–30 kDa showed the most tumor growth inhibitory effect despite the fact that its sulfate content is lower than those in the F5 kDa and F30 kDa fractions.
Biological and Physiological Functions of Fucoidan
In its natural role, fucoidan is a cell wall component responsible for maintaining the moist condition of brown seaweeds and also for protecting them against microorganisms (Kloareg and Quatrano, 1988; Bisgrove and Kropf, 2001; Mabeau et al., 1990). It has been found that the fucoidan content is higher when the seaweeds grow in deeper water, dem- onstrating its important effect in the protection of seaweeds (Black, 1954; Evans, 1989). Similar to other natural substances, the high bioactivities and low toxicity of fucoidan have caught attention from health-related industries in search of novel health-benefitting sub- stances. It is known that fucoidan has a cytotoxic effect on tumor cells but not on normal cells (Yan et al., 2015). Fucoidan can indirectly kill cancer cells via enhancing immunity through activating natural killer (NK) cells and macrophages. It can also arrest cell cycle, especially the G1 phase, inducing apoptosis and antiangiogenesis and inhibiting prolifer- ative cells. Clinically, fucoidan can exert both preventive and therapeutic roles but exhibits no liver toxicity and do not affect liver function. The many bioactivities of fucoidan are summarized in the following sections.
1. Anticoagulant Activity
The ability of sulfated polysaccharides to interfere with biological systems has been illus- trated with heparin, which is a highly sulfated polysaccharide widely used for antico- agulant therapy. As a sulfated polysaccharide extracted from brown seaweeds, fucoidan demonstrated excellent anticoagulant activity and huge potential for drug development. It can inhibit the activities of coagulation factors via interaction with antithrombin in the coagulation pathways (Jung et al., 2007). It has been found that the anticoagulant activ- ity is positively correlated to the molecular weight of fucoidan, with the content of sulfate groups also positively affecting the anticoagulant activities (Kitamura et al., 1991; Dobashi et al., 1989).
2. Antitumor/Anticancer Activity
Fucoidan is known to possess antitumor/anticancer activities. Han et al. (2008) found that fucoidan exhibited a significant anticancer activity against human lung cancer cell line (A549) and gastric cancer cell line (AGS). A large number of studies have shown that over- sulfated fucoidan in brown seaweeds exhibits antiproliferative activity by inhibiting the angiogenesis around the tumor cells and blocking the infiltration and metastasis of the tumor cells (Matou et al., 2002; Koyanagi et al., 2003). Their results indicated that the over- sulfated fucoidan induced apoptosis via MAPK-dependent pathways. Both natural and oversulfated fucoidans significantly suppressed the mitogenic and chemotactic actions of vascular endothelial growth factor 165 (VEGF165) on HUVEC by preventing the binding of VEGF165 to its cell surface receptor. The oversulfated fucoidan has also been found to exert remarkable growth-inhibitory activities on Sarcoma-180 cells and possess antitumor activity against L-1210 leukemia cells in mice. The antitumor activity of fucoidan in PC-3, HeLa, and HepG2 cancer cells is in similar pattern, where results demonstrated that sulfa- tion played an essential role in antitumor activity. Takeda et al. (2012) reported that the antitumor activity of fucoidan in Sarcoma-180 cells is mediated through increasing NO production by fucoidan-stimulated macrophages via nuclear factor–dependent signaling pathway. Fucoidan exerted a potent inhibitory effect on EGF-induced phosphorylation of epi- dermal growth factor receptor (EGFR), which is one of the receptor tyrosine kinases and plays an important role in regulating cell proliferation, differentiation, and transforma- tion. Nagamine et al. (2009) demonstrated that fucoidan modulates the expression of che- mokine ligand 12 (CXCL12)/chemokine receptor 4 (CXCR4) and exerts antitumor activity in Huh7 hepatoma cells. Yang et al. (2008) confirmed that fucoidan isolated from Undaria pinnatifida induced apoptosis of mouse liver cancer cells in vitro via downregulation of apoptosis protein Survivin and Bel-2 expression. Koyanagi et al. (2003) showed that the antitumor effect of fucoidan is due to its antiangiogenesis activity.
Immune regulation is one of the main mechanisms of many antitumor effects. Maruyama et al. (2006) studied the effects of dietary Mekabu fucoidan on the tumor growth of mouse A20 leukemia cells and on T cell–mediated immune responses in T cell recep- tor transgenic mice. Results suggested that Mekabu fucoidan mediated tumor destruction through Th1 cell and NK cell response.
3. Immunoregulation Activity
As a biological immunomodulator, fucoidan can activate the body’s immunologi- cal defense system and suppress the development of tumor cells through enhancing the body’s immunomodulatory activity (Maruyama et al., 2006). As mentioned ear- lier, fucoidan can significantly enhance the cytolytic activity of NK cells by increasing macrophage-mediated immune response signaling molecules, such as interleukins IL-2 and IL-12 and IFN-γ. Okai et al. (1996) found that fucoidan caused stimulatory effects on the ingestive activity of mouse phagocytic cells against Staphylococcus aureus and the release of cytokines, IL-1 alpha and tumor necrosis factor alpha (TNF-α), from the same cells. Furthermore, fucoidan enhanced polyclonal antibody (IgM and IgG) pro- duction in spleen cells. Han et al. (2008) found that fucoidan can significantly improve the normal and immunosuppressed mice spleen cell growth in the cyclophosphamide immunocompromised mouse model. In a mouse model, Cladosiphon-derived fucoidan downregulated IL-6 (a Th2 cytokine) and ameliorated colitis (Matsumoto et al., 2004). Other studies in mice have also observed significant immunomodulatory effects by increasing the activity of NK cells and via a modulation of the Th1:Th2 ratio (Maruyama et al., 2005).
4. Antioxidant Activity
Fucoidan is an excellent natural antioxidant. It has exhibited significant antioxidant activi- ties in vitro and has great prospects for the prevention of diseases induced by free radicals. In animal studies, fucoidan improved the antioxidant defense system in treated animals and considerably reduced the oxidative stress exerted by isoproterenol. A recent finding suggested that fucoidan had a protective effect on 1-methyl-4-phenyl-1,2,3,6-tetrahydro- pyridine (a neurotoxin)–induced neurotoxicity in Parkinson disease via its antioxidant activity (Luo et al., 2009). It is also reported that fucoidan has the antioxidant capacity to prevent kidney damage caused by excessive free radicals and improve the microenviron- ment to inhibit stone formation (Veena et al., 2006, 2007).
5. Antiviral Activity
As a sulfated polysaccharide, fucoidan can intervene in the adsorption of virus to host cells, inhibit viral reverse transcriptase, prevent syncytium formation, and play an impor- tant role in antiviral activities. Lee et al. (2004) found that fucoidan extracted from U. pin- natifida can protect mice from herpes simplex virus (HSV) infection and directly inhibit viral replication through enhancing innate and acquired immune defenses. Fucoidan extracted from Adenocystis utricularis, Stoechospermum marginatum, Cystoseira indica, and U. pinnatifida also showed antiviral activity against both HSV-1 and HSV-2. Hidari et al. (2008) reported that fucoidan can effectively inhibit the dengue virus type 2 (DEN2) infection. It was found that DEN2 particles bound exclusively to fucoidan, indicating that fucoidan interacts directly with envelope glycoprotein (EGP) on DEN2. Structure-based analysis suggested that Arg323 of DEN2 EGP, which is conformationally proximal to one of the putative heparin-binding residues, Lys310, is critical for the interaction with fucoidan. It was concluded that both the sulfated group and glucuronic acid of fucoidan account for the inhibition of DEN2 infection.
The antiviral mechanism of fucoidan may be due to the negative charge on its sulfated polysaccharide structure, allowing it to bind to the positively charged regions on the outer membrane glycoprotein of the virus, thereby resulting in the shielding of this region and inhibiting the virus from binding to host cells. Fucoidan is structurally similar to glycos- aminoglycans on the cell surface and can inhibit viral and cellular binding in a competitive manner, thus hindering virus adsorption. In a study on the interaction of the transactivat- ing protein HIV-1 with sulfated polysaccharides, it was found that the antiviral activity of fucoidan is primarily mediated by blocking the binding of HIV to the receptor CD4 on the target cell (Waston et al., 1999).
6. Antiinflammatory Activity
Inflammation is a defensive response of a living tissue with a vascular system to an injury factor. The inflammatory process involves a series of events that can be elicited by numerous internal or external stimuli. Antiinflammatory refers to the property of a substance or treatment that reduces inflammation. Fucoidan is known for having antiinflammatory activities. It is a potent modulator of connective tissue proteolysis. In addition, it can be used for treating inflammatory pathologies in extracellular matrix, which is important because connective tissue destruction during inflammatory dis- eases, such as chronic wound, chronic leg ulcers, or rheumatoid arthritis, is the result of continuous supply of inflammatory cells and exacerbated production of inflamma- tory cytokines and matrix proteinases (Senni et al., 2006). Mizuno et al. (2009) found that fucoidan can stimulate the RAW 264.7 cells to produce TNF-α, thereby inhibiting the mRNA expression of interleukin in Caco-2 cells. It was reported that treatment with fucoidan attenuated the increased plasma concentrations of inflammatory cytokines, including TNF-α and IL-6 in rats with ischemia/reperfusion-induced myocardial dam- age (Li et al., 2011).
7. Antihypertensive and Hypoglycemic Activity
High-plasma cholesterol levels and high blood pressure are causes of cardiovascular disease. Seaweed-derived polysaccharides such as alginate, carrageenan, funoran, laminaran, por- phyran, ulvan, and fucoidan are able to produce hypocholesterolemic and hypolipidemic responses owing to reduced cholesterol absorption in the gut (Panlasigui et al., 2003). In par- ticular, fucoidan has antihypertensive and hypoglycemic effects because it can improve islet cell morphology and its function, accelerate insulin secretion, and promote the use of sugar of peripheral tissues such as liver and muscles. In addition, it can also affect the enzyme activities of glycometabolism and reduce blood glucose level. It was found that fucoidan has a strong ability of removing reactive oxide and being able to reduce blood lipid levels of hyperlipidemia mice (Li et al., 1999, 2001). In addition, it was found that fucoidan can reduce serum triglycer- ides by activating serum and liver lipoprotein lipase and hepatic lipase and also reduce serum cholesterol by activating lecithin–cholesterol acyltransferase. Fucoidan can significantly increase the expression of low-density lipoprotein (LDL)-mRNA and promote LDL-cholesterol (LDL-C) removal, which plays an important role in reducing serum cholesterol.
Hyperlipidemia and lipid metabolism disorders are the main risk factors of atheroscle- rosis, which can lead to vascular stenosis and occlusion and even cause a series of cardio- vascular and cerebrovascular diseases. The complex structure of fucoidan is similar to that of sialic acid, which can increase negative charge on the cell surface, with the resultant formation of electrostatic repulsion among cells being able to prevent the deposition and promote the decomposition and excretion of cholesterol.
Park et al. (2011) used 3T3-L1 adipocytes and studied the effect of fucoidan on the inhibition of lipid accumulation. It was found that fucoidan (200 μg/mL) decreased lipid accumulation by 52%, reduced triglyceride deposition by 15%, and increased hor- mone-sensitive lipase expression. Fucoidan (100 μg/mL) from U. pinnatifida suppressed adipogenesis in 3T3-L1 cells via downregulating the mRNA gene expression of key adip- ogenic markers such as peroxisome proliferator–activated receptor-γ (PPAR-γ), CCAAT/ enhancer-binding protein-α (C/EBPα), and adipocyte protein-2 (aP2). Fucoidan (100 μg/ mL) also inhibited adipocyte differentiation and thereby prevented lipid accumulation in 3T3-L1 cells by suppressing mRNA gene expressions of key adipocyte differentiation markers such as TNF-α, monocyte chemotactic protein-1 (MCP-1), and plasminogen acti- vator inhibitor-1 (PAI-1) (Kim and Lee, 2012).
8. Gastric Protective Effect
Fucoidan is able to adsorb Helicobacter pylori in the stomach and discharge it from the digestive system. It can reduce the incidence of gastritis, gastric ulcer, and gastric can- cer and has excellent gastric mucosal protective effect. Shibata et al., (2000) investigated the antipeptic activity, basic fibroblast growth factor stabilizing activity, and inflammatory properties of fucoidan to elucidate its antiulcer potential. Results showed that antipeptic activity was observed with fucoidan and other sulfated polysaccharides such as dextran sulfate and carrageenan, but nonsulfated polysaccharides such as mannan and dextran did not exert the antipeptic activity. The loss of bFGF bioactivity was prevented by all sul- fated polysaccharides tested except chondroitin sulfate, at pH 7.4 and pH 4.0. Overall, the results suggest that fucoidan is a safe substance with potential for gastric protection.
Health Benefits and Potential Applications of Fucoidan
In recent years, marine-derived functional food ingredients have been recognized for their vital role in human health and nutrition. The possibilities of designing new functional foods and nutraceuticals from marine products are promising, and many marine-derived ingredients such as fucoidan have been used as active ingredients for the preparation of functional foods and nutraceutical products. A wide range of biological activities associ- ated with the natural compounds derived from marine sources have potential to expand the health beneficial value not only in the food industry but also in the nutraceutical and pharmaceutical industries.
Fucoidan has a highly sulfated chemical structure that appears to be responsible for many demonstrated biological activities in vitro (Ustyuzhanina et al., 2014). In the recent years, there has been a significant amount of development work for fucoidan in its appli- cations in pharmaceutical, nutraceutical, cosmeceutical, and functional food industries. There is a growing interest among manufacturers and the consumers in these areas look- ing for novel health-enhancing benefits from products of natural origin. In this respect, research during the past decade has provided extensive scientific evidence for the health benefits of brown seaweed–derived fucoidan, which has shown antitumor, immunomod- ulatory, antiinflammatory, and many other bioactivities. In South Korea, Japan, Australia, France, the United States, and the United Kingdom, fucoidan is already commercially available as health supplements, made into products in the forms of beverages, capsules, and tablets, which are intended to enhance the physical health, defend the immune sys- tem, and, overall, improve the well-being of many consumers.
Summary
Fucoidan is a sulfated polysaccharide extracted from brown seaweeds. It has anticoagu- lant, antiinflammatory, antitumor, antioxidant, and many other novel bioactivities. As a marine-derived natural food ingredient, the biological functions of fucoidan are closely related to its chemical structure, which is again dependent on the species of seaweeds, the extraction conditions, and the chemical and physical treatment of this novel compound. Owing to its many health-benefitting properties, fucoidan is now widely used in the pro- duction of functional food products.
References
Albuquerque, I.R.L., Queiroz, K.C.S., Alves, L.G., Santos, E.A., Leite, E.L., Rocha, H.A.O., 2004. Heterofucans from Dictyota menstrualis have anticoagulant activity. Brazilizan Journal of Medical and Biological Research 37, 167–171.
Ale, M.T., Meyer, A.S., 2013. Fucoidans from brown seaweeds: an update on structures, extraction tech- niques and use of enzymes as tools for structural elucidation. RSC Advances 3, 8131–8141.
Ale, M.T., Mikkelsen, J.D., Meyer, A.S., 2011. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysac- charides from brown seaweeds. Marine Drugs 9, 2106–2130.
Atashrazm, F., Lowenthal, R.M., Woods, G.M., Holloway, A.F., Dickinson, J.L., 2015. Fucoidan and cancer: a multifunctional molecule with anti-tumor potential. Marine Drugs 13, 2327–2346.
Bagasra, O., Whittle, P., Hein, B., Pomerantz, R.J., 1991. Anti-human immunodeficiency virus type 1 activity of sulfated monosaccharides comparison with sulfated polysaccharide and other polyions. Journal of Infections Diseases 164 (6), 1082–1088.
Bilan, M.I., Grachev, A.A., Ustuzhanina, N.E., Shashkov, A.S., Nifantiev, N.E., Usov, A.I., 2002. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydrate Research 337, 719–730.
Bilan, M.I., Grachev, A.A., Shashkov, A.S., Nifantiev, N.E., Usov, A.I., 2006. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydrate Research 341, 238–245.
Bilan, M.I., Grachev, A.A., Ustuzhanina, N.E., Shashkov, A.S., Nifantiev, N.E., Usov, A.I., 2004. A highly reg- ular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydrate Research 339, 511–517.
Bisgrove, S.R., Kropf, D.L., 2001. Cell wall deposition during morphogenesis in fucoid algae. Planta 212, 648–658.
Black, W.A.P., 1954. The seasonal variation in the combined L-fucose content of the common British lami- nariaceae and fucaceae. Journal of the Science of Food and Agriculture 5 (9), 445–448.
Black, W.A.P., Cornhill, W.J., Dewar, E.T., Woodward, F.N., 1953. Manufacture of algal chemicals. VI.- Laboratory-scale isolation of l-fucose from brown marine algae. Journal of the Science of Food and Agriculture 1953, 81–91.
Catarina, O., Ferreira, A.S., Novoa-Carballal, R., Nunes, C., Pashkuleva, I., Neves, N.M., Coimbra, M.A., Reis, R.L., Martins, A., Silva, T.H., 2017. The key role of sulfation and branching on fucoidan antitumor activ- ity. Macromolecular Bioscience 17 (5), 121–128.
Cho, M.L., Lee, B.Y., You, S.G., 2010. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules: a Journal of Synthetic Chemistry and Natural Product Chemistry 16, 291–297.
Choi, Y., Hosseindoust, A., Goel, A., Lee, S., Jha, P.K., Kwon, I.K., Chae, B.J., 2017. Effects of Ecklonia cava as fucoidan-rich algae on growth performance, nutrient digestibility, intestinal morphology and caecal microflora in weanling pigs. Asian-Australas Journal of Animal Sciences 30 (1), 64–70.
Conchie, J., Percival, E.G.V., 1950. Fucoidin. Part II. The hydrolysis of a methylated fucoidin prepared from
Fucus vesiculosus. Journal of the Chemical Society 1950, 827–832.
Cumashi, A., Ushakova, N.A., Preobrazhenskaya, M.E., D’Incecco, A., Piccoli, A., Totani, L., Tinari, N., Morozevich, G.E., Berman, A.E., Bilan, M.I., Usov, A.I., Ustyuzhanina, N.E., Grachev, A.A., Sanderson, C.J., Kelly, M., Rabinovich, G.A., Iacobelli, S., Nifantiev, N.E., 2007. A comparative study of the anti- inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 17 (5), 541–552.
Dobashi, K., Nishino, T., Fujihara, M., Nagumo, T., 1989. Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from seaweed Hizikia fusiforme. Carbohydrate Research 194 (1), 315–320.
Evans, L.V., 1989. Mucilaginous substances from macroalgae: an overview. Symposia of the Society for Exprime Biological 43, 455–461.
Ferial, H.B., Ellouali, M., Sinquin, C., Boisson-Vidal, C., 2000. Relationship between sulfate groups and biological activities of fucans. Thrombosis Research 100, 453–459.
Hahn, T., Zayed, A., Kovacheva, M., Stadtmüller, R., Lang, S., Muffler, K., Ulber, R., 2016. Dye affinity chro- matography for fast and simple purification of fucoidan from marine brown algae. Engineering in Life Sciences 16 (1), 78–87.
Han, J.G., Syed, A.Q., Kwon, M., Ha, J.H., Lee, H.Y., 2008. Antioxidant, immunomodulatory and anticancer activity of fucoidan isolated from Fucus vesiculosus. Journal of Biotechnology 136 (4), S571–S578.
Hidari, K.I., Takahashi, N., Arihara, M., Nagaoka, M., Morita, K., Suzuki, T., 2008. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochemical and Biophysical Research Communications 376 (1), 91–95.
Jung, W.K., Athukorala, Y., Lee, Y.J., Cha, S.H., Lee, C.H., Vasanthan, T., Choi, K.S., Yoo, S.H., Kim, S.K., Jeon, Y.J., 2007. Sulfated polysaccharide purified from Ecklonia cava accelerates antithrombin III-mediated plasma proteinase inhibition. Journal of Applied Phycology 19, 425–430.
Katsumi, A., Yokoyama, T., Matsuo, K., 2016. Structural characteristics and antioxidant activities of fucoid- ans from five brown seaweeds. Journal of Applied Glycoscience 63, 31–37.
Kitamura, K., Matsuo, M., Yasui, T., 1991. Fucoidan from brown seaweed Laminaria angustata var. longis- sima. Agriculture Biological Chemistry 55 (2), 615–616.
Kim, K.J., Lee, B.Y., 2012. Fucoidan from the sporophyll of Undaria pinnatifida suppresses adipocyte differ- entiation by inhibition of inflammation-related cytokines in 3T3-L1 cells. Nutrition Research 32, 439–447.
Kimiko, A., Terahata, H., Hayashi, Y., Seno, N., 1966. Isolation and purification of fucoidin from brown seaweed Pelvetia wrightii. Biological Chemistry 30 (5), 495–499.
Kloareg, B., Quatrano, R.S., 1988. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanography and Marine Biology – An Annual Review 26, 259–315.
Koyanagi, S., Tanigawa, N., Nakagawa, H., 2003. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochemical Pharmacology 65 (2), 173–179.
Kylin, H., 1913. Biochemistry of sea algae. Zeitschrift für Physikalische Chemie 83, 171–197.
Lee, J.B., Hayashi, K., Hashimoto, M., Nakamo, T., Hayashi, T., 2004. Novel antiviral fucoidan from sporo- phyll of Undaria pinnatifida (Mekabu). Chemical Pharmaceutiacal Bulletin 52 (9), 1091–1094.
Li, C., Gao, Y., Xing, Y., Zhu, H., Shen, J., Tian, J., 2011. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia–reperfusion injury in rats via regulating the inflammation response. Food and Chemical Toxicology 49, 2090–2095.
Li, B., Wei, X.J., Sun, J.L., Xu, S.Y., 2006. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed, Hizikia fusiforme. Carbohydrate Research 341, 1135–1146.
Li, D.Y., Xu, Z., Huang, L.M., Wang, H.B., Zhang, S.H., 2001. Effect of fucoidan of L. japonica on rats with hyperlipidaemia. Food Science 22, 92–95.
Li, D.Y., Xu, Z., Zhang, S.H., 1999. Prevention and cure of fucoidan of L. japonica on mice with hypercho- lesterolemia. Food Science 20, 45–46.
Lionel, C., Mulloy, B., Ratiskol, J., Colliec-Jouault, S., 2001. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydrate Research 330, 529–535.
Luo, D., Zhang, Q., Wang, H., Cui, Y., Sun, Z., Yang, J., Zheng, Y., Jia, J., Yu, F., Wang, X., Wang, X., 2009. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. European Journal of Pharmacology 617, 33–40.
Mabeau, S., Kloareg, B., Joseleau, J.P., 1990. Fractionation and analysis of fucans from brown algae.
Phytochemistry 29, 2441–2445.
Magdel-Din Hussein, M., Abdel-Aziz, A., Mohamed-Salem, H., 1980. Sulfated heteropolysaccharides from
Padina pavoia. Phytochemistry 19, 2131–2132.
Manish, S.P., Oehninger, S., Townsend Barnett, Q., Williams, R.L., Clark, G.F., 1993. A revised structure for fucoidan may explain some of its biological activities. The Journal of Biological Chemistry 268 (29), 21770–21776.
Marais, M.F., Joseleau, J.P., 2001. A fucoidan fraction from Ascophyllum nodosum. Carbohydrate Research 336, 155–159.
Maria, E.R.D., Cardoso, M.A., Noseda, M.D., Cerezo, A.S., 2001. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydrate Research 333, 281–293.
Maruyama, H., Tamauchi, H., Hashimoto, M., Nakano, T., 2005. Suppression of Th2 immune responses by Mekabu fucoidan from Undaria pinnatifida sporophylls. International Archives of Allergy and Immunology 137 (4), 289–294.
Maruyama, H., Tamauchib, H., Iizuka, M., Nakano, T., 2006. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Medica 72, 1415–1417.
Matou, S., Helley, D., Chabut, D., 2002. Effect of fucoidan on fibroblast growth factor-2-induced angiogen- esis in vitro. Thrombosis Research 106 (4–5), 213–221.
Matsumoto, S., Nagaoka, M., Hara, T., Kimura-Takagi, I., Mistuyama, K., Ueyama, S., 2004. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regula- tion of interleukin-6 production on colonic epithelial cells. Clinical and Experimental Immunology 136 (3), 432–439.
McCulure, M.O., Whitby, D., Patience, C., Cheinson-Popov, R., Weber, J.N., 1991. Dextran sulfate and fucoidan are potent inhibitors of HIV infection in vitro. Antiviral Chemistry and Chemotherapy 2, 19–26.
Mian, A.J., Percival, E., 1973. Carbohydrates of the brown seaweeds himanthalia lorea and bifurcaria bifur- cata. Carbohydrate Research 26, 147–161.
Mizuno, M., Nishitani, Y., Hashimoto, T., 2009. Different suppressive effects of fucoidan and lentinan on IL-8 mRNA expression in in vitro gut inflammation. Bioscience, Biotechnology, and Biochemistry 73 (10), 2324–2325.
Mulloy, B., Ribeiro, A., Alves, A.P., Vieira, R.P., Moural, P.A.S., 1994. Sulfated fucans from Echinoderms have a regular tetrasaccharide repeating unit defined by specific patterns of sulfation at the 0-2 and 0-4 positions. Journal of Biological Chemistry 269 (35), 22113–22123.
Nagamine, T., Hayakawa, K., Kusakabe, T., 2009. Inhibitory effect of fucoidan on Huh7 hepatoma cells through downregulation of CXCL12. Nutrition and Cancer 61 (3), 340–347.
Okai, Y., Ishizaka, S., Higashi-Okai, K., 1996. Detection of immunomodulating activities in an extract of Japanese edible seaweed, Laminaria japonica (Makonbu). Journal of the Science of Food and Agriculture 72 (4), 455–460.
Panlasigui, L.N., Baello, O.Q., Dimatangal, J.M., Dumelod, B.D., 2003. Blood cholesterol and lipid- lowering effects of carrageenan on human volunteers. Asia Pacific Journal of Clinical Nutrition 12, 209–214.
Park, M.K., Jung, U., Roh, C., 2011. Fucoidan from marine brown algae inhibits lipid accumulation. Marine Drugs [Electronic Resource] 9, 1359–1367.
Percival, E., 1968. Glucoroxylofucan, a cell-wall component of Ascophyllum nodosum. Carbohydrate Research 7, 272–283.
Ponce, N.M., Pujol, C.A., Damonte, E.B., Flores, M.L., Stortz, C.A., 2003. Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydrate Research 338 (2), 153–165.
Rocha, H.A.O., Moraes, F.A., Trindade, E.S., Franco, C.R.C., Torquato, R.J.S., Veiga, S.S., Valente, A.P., Mourao, P.A.S., Leite, E.L., Nader, H.B., Dietrich, C.P., 2005. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schroederi: an ideal antithrombotic agent? Journal of Biological Chemistry 280, 41278–41288.
Senni, K., Gueniche, F., Foucault-Bertaud, A., Igondjo-Tchen, S., Fioretti, F., Colliec-Jouault, S., Durand, P., Guezennec, J., Godeau, G., Letourneur, D., 2006. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Archives of Biochemistry and Biophysics 445, 56–64.
Shibata, H., Kimura-Takagi, I., Nagaoka, M., Hashimoto, S., Aiyama, R., Iha, M., Ueyama, S., Yokokura, T., 2000. Properties of fucoidan from Cladosiphon okamuranus tokida in gastric mucosal protection. Biofactors 11, 235–245.
Silchenko, A., Kusaykin, M., Kurilenko, V., Zakharenko, A., Isakov, V., Zaporozhets, T., Gazha, A., Zvyagintseva, T., 2013. Hydrolysis of fucoidan by fucoidanase isolated from the marine bacterium, formosa algae. Marine Drugs 11, 2413–2430.
Silva, T.M.A., Alves, L.G., Santos, M.G.L., de Queiroz, K.C.S., Marques, C.T., Chavante, S.F., Rocha, H.A.O., Leite, E.L., 2005. Partial characterization and anticoagulant activity of a heterofucan. Brazilian Medical and Biological Research 38, 523–533.
Takashi, N., Nishioka, C., Ura, H., Nagumo, T., 1994. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydrate Research 255, 213–224.
Takashi, N., Yokoyama, G., Dobashi, K., Fujihara, M., Nagumo, T., 1989. Isolation, purification, and char- acterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia Kurome and their blood-anticoagulant activities. Carbohyrate Research 186, 119–129.
Takeda, K., Tomimori, K., Kimura, R., Ishikawa, C., Nowling, T.K., Mori, N., 2012. Anti-tumor activity of fucoidan is mediated by nitric oxide released from macrophages. International Journal of Oncology 40, 251–260.
Tissot, B., Salpin, J.Y., Martinez, M., Gaigeot, M.P., Daniel, R., 2006. Differentiation of the fucoidan sul- fated l-fucose isomers constituents by CE-ESIMS and molecular modeling. Carbohydrate Research 341, 598–609.
Ustyuzhanina, N.E., Bilan, M.I., Ushakova, N.A., Usov, A.I., Kiselevskiy, M.V., Nifantiev, N.E., 2014.
Fucoidans: pro- or antiangiogenic agents? Glycobiology 24, 1265–1274.
Veena, C.K., Josephine, A., Preetha, S.P., Varalakshmi, P., Sundarapandiyan, R., 2006. Renal peroxidative changes mediated by oxalate: the protective role of fucoidan. Life Sciences 79, 1789–1795.
Veena, C.K., Josephine, A., Preetha, S.P., Varalakshmi, P., 2007. Physico-chemical alterations of urine in experimental hyperoxaluria: a biochemical approach with fucoidan. The Journal of Pharmacy and Pharmacology 59, 419–527.
Waston, K., Gooderham, N.J., Davies, D.S., 1999. Interaction of the transactivating protein HIV-1 with sul- fated polysaccharides. Biochemical Pharmacology 57, 775–783.
Xue, C.H., Fang, Y., Lin, H., Chen, L., Li, Z.J., Deng, D., Lu, C.X., 2001. Chemical characters and antioxida- tive properties of sulfated polysaccharides from Laminaria japonica. Journal of Applied Phycology 13 (1), 67–70.
Yan, M.D., Yao, C.J., Chow, J.M., Chang, C.L., Hwang, P.A., Chuang, S.E., Whang-Peng, J., Lai, G.M., 2015. Fucoidan elevates microRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human hepa- tocellular carcinoma cells. Marine Drugs 13, 6099–6116.
Yang, C., Chung, D., Shin, I.S., Lee, H.Y., Kim, J.C., Lee, Y.J., You, S.G., 2008. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. International Journal of Biological Macromolecules 43, 433–437.
Further Reading
Andrade, L.R., Salgado, L.T., Farina, M., Pereira, M.S., Mourão, P.A.S., Filho, G.M.A., 2004. Ultrastructure of acidic polysaccharides from the cell walls of brown algae. Journal of Structural Biology 145, 216–225.
Senthilkumar, K., Manivasagan, P., Venkatesan, J., Kim, S.K., 2013. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. International Journal of Biological Macromolecules 60, 366–374.
Company: SeaHerb Co., LTD.
CEO: Oh, Chungheon
Address: 276, Solchi-ro, Baebang-eup, Asan-si, Chungcheongnam-do, S.Korea
CS Center: mekabu@naver.com
Tel: +82 41-549-6466
Email: sh@seaherb.com
www.seaherb.com