
Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer
Kalimuthu Senthilkumara, Panchanathan Manivasagana, Jayachandran Venkatesana, Se-Kwon Kima,
a Marine Bioprocess Research Center, Pukyong National University, Busan 608-737, Republic of Korea
b Department of Chemistry, Pukyong National University, Busan 608-737, Republic of Korea
a r t i c l e i n f o
Article history:
Received 3 May 2013
Received in revised form 19 June 2013 Accepted 22 June 2013
Available online 28 June 2013
a b s t r a c t
Seaweeds, being abundant sources of bioactive components have much interest in recent times. The complex polysaccharides from the brown, red and green seaweeds possess broad spectrum therapeu- tic properties. The sulfated polysaccharides are routinely used in biomedical research and have known biological activities. Fucoidan, a fucose-rich polysaccharide extracted from brown seaweed has vari- ous biological functions including anticancer effects. Cellular damage induces growth arrest and tumor suppression by inducing apoptosis, the mechanism of cell death depends on the magnitude of DNA damage following exposure to anticancer agents. Apoptosis is mainly regulated by cell growth signaling molecules. Number of research studies evidenced that fucoidan shown to induce cytotoxicity of vari- ous cancer cells, induces apoptosis, and inhibits invasion, metastasis and angiogenesis of cancer cells. There are few articles discussing on fucoidan biological activity but no specific review on cancer and its signaling mechanism. Hence, this review discusses the brown seaweed fucoidan structure and some bio- logical function and role in apoptosis, invasion, metastasis, angiogenesis and growth signal mechanism on cancer.
Life began in the sea, and oceans, particularly rich in biodiver- sity, cover over 70% of the Earth’s surface. The marine environment contains a diverse number of plants, animals, and microorganisms, which have a wide diversity of natural products [1]. The inability to cure many diseases including cancer has encouraged the need for the development of new drugs from natural sources. Among the natural sources, marine environment has great frontier for pharmaceutical and medical research. Recent studies in the field of cancer research have revealed promising compounds, isolated from natural sources, with proven anticancer activity. Seaweeds have great potential as a supplement in functional food or for the extraction of compounds. Seaweeds are known for their richness in polysaccharides, minerals and certain vitamins, but they also con- tain bioactive substances like polysaccharides, proteins, lipids and polyphenols, with antibacterial, antifungal, antiviral properties, etc. [2,3] and used in the development of new pharmaceutical agents [4]. The Phaeophyceae or brown algae, is a large group of mostly marine multicellular algae, including of many seaweeds. They play an important role in marine environments, both as food and for the habitats they form. There are number of studies evidenced that anticancer effects of fucoidans, but no review available to speculate the fucoidan anticancer action. The scope of this review we discusses the role of fucoidan on biological activity, apoptosis, metastasis, angiogenesis and growth signal regulation on cancer
Marine algae contain large amounts of polysaccharides, notably cell wall structural, also mycopolysaccharides and storage polysac- charides [2,5]. Polysaccharides are polymers of simple sugars (monosaccharides) linked together by glycosidic bonds, and they have numerous commercial applications in products such as sta- bilizers, thickeners, emulsifiers, food, feed and beverages [6,7]. The total polysaccharide concentrations in the seaweed species of interest range from 4% to 76% of dry weight [3]. Seaweeds have low lipid, high carbohydrate and more dietary fibers. However, dietary fibers are good for human health. The cell wall polysac- charides mainly consist of cellulose and hemicelluloses, neutral polysaccharides, and are thought to physically support the thal- lus in water. The cell wall and storage polysaccharides are species specific. Green algae contain sulphuric acid polysaccharides, sul- phated galactans and xylans, brown algae contains alginic acid, fucoidan (sulphated fucose), laminarin (β-1,3 glucan) and sargas- san and red algae contains agars, carrageenans, xylans, floridean starch (amylopectin-like glucan), water-soluble sulphated galac- tan, as well as porphyran as mucopolysaccharides located in the intercellular spaces [2,5]. The components of galactose, glucose, mannose, fructose, xylose, fucose and arabinose were found in the total sugars in the hydrolysates. The glucose content was 65%, 30% and 20% of the total sugars in an autumn sample of 50 individual plants of Saccharina, Fucus (serratus and spiralis) and Ascophyllum, respectively [8]. Several other polysaccharides are present in and utilized from seaweed, e.g., furcellaran, funoran, ascophyllan and sargassan.
In recent years, much attention has been focused on polysac- charides isolated from natural sources. During the last decade, numerous bioactive polysaccharides with interesting functional properties has discovered from seaweeds [9]. Fucoidan is a sulfated polysaccharide (MW: average 20,000) found mainly in various species of brown algae and brown seaweed such as mozuku, kombu, limu moui, bladderwrack, wakame, and hijiki (variant forms of fucoidan have also been found in animal species, including the sea cucumber). Fucoidan is a term used for a class of sulfated, fucose rich, polysaccharides found in the fibrillar cell walls and intercellular spaces of brown seaweeds. FCSPs (Fucoidan contain- ing sulfated polysaccharides) may also contain galactose, mannose, xylose, glucose and/or glucuronic acid, usually in minor amounts [10]. The polysaccharide was named as “fucoidin” when it was first isolated from marine brown algae by Kylin in 1913 [11]. Now it is named as “fucoidan” according to IUPAC rules, but also called as fucan, fucosan or sulfated fucan [12]. Fucoidan is considered as a cell wall-reinforcing molecule and seems to be associated with protection against the effects of desiccation when the seaweed is exposed at low tide. Fucoidans from several species of brown seaweed, for example Fucus vesiculosus, have simple chemical compositions, mainly being composed of fucose and sulfate. But the chemical compositions of most fucoidans are complex.
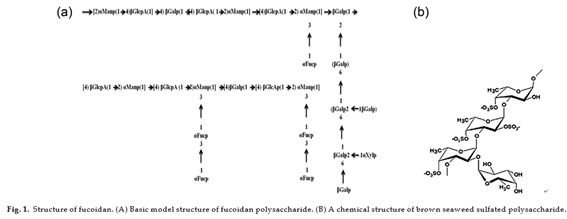
The major components of fucoidan are l-fucose and sul- fate content. The most studied FCSPs, originally called fucoidin, fucoidan or just fucans, have a backbone built of (1 3)-linked α-l- fucopyranosyl residues or of alternating (1 3)- and (1 4)-linked α-l-fucopyranosyl residues [13,14]. These fucopyranosyl residues may be substituted with short fucoside side chains or sulfate groups at C-2 or C-4, and may also carry other minor substitutions, e.g., acetate, xylose, mannose, glucuronic acid, galactose, or glucose [15–17]. FCSPs also include sulfated galactofucans with backbones built of (1 6)-β-d-galacto- and/or (1 2)-β-d-mannopyranosyl units. In addition to sulfate these backbone residues may be substi- tuted with fucosides, single fucose substitutions, and/or glucuronic acid, xylose or glucose substitutions [16]. Fucoidan extracted from brown seaweed algae Fucus vesiculosus [18], Ascophyllum nodosum [19], Sargassum kjellmanianum [20], Sargassum thunbergii [21], Cladosiphon okamuranus Tokida [22], in which the percentage of l-fucose ranged from 12.6 to 36.0%, and the percentage of sulfate content from 8 to 25%. The structure of fucoidan is depicted in Fig. 1.
Recently studies suggested that low molecular weight fucoidan (LMWF) has more biological actions than native fucoidan. LMWF, a sulfated polysaccharide derived from brown seaweeds. The pharmacological effects of fucoidans vary with their molecular weight, which is generally classified as low (<10 kDa), medium (10–10,000 kDa), or high >10,000 kDa [23]. LMWF mediated the broad-spectrum growth inhibition of human carcinoma cells, including HeLa cervix adenocarcinoma, HT1080 fibrosarcoma, K562 leukemia, U937 lymphoma, A549 lung adenocarcinoma and HL-60 [24]. LMWF inhibits the invasion and angiogenesis of HT 1080 fibrosarcoma cells and induces apoptosis in MCF-7 cancer cells via mitochondria mediated pathway [24,25]. LMWF induces apoptosis through mitochondrial mediated pathways in MDA-MB- 231 breast cancer cells and also evidenced that the interrelated roles of Ca2+ homeostasis, mitochondrial dysfunction and caspase activation [26]. LMWF has sulfate content higher than 20% was found to exert profound anticoagulant activity as well as antipro- liferative effects on fibroblast cell line (CCL39) in a dose-dependent fashion [27].
LMWF has anti-inflammatory properties such as anti- complementary activities with both inhibition of leukocyte accumulation and connective tissue proteolysis. LMWF could be used for treating some inflammatory diseases in which uncontrolled extracellular matrix degradation [28]. LMWF can also promote tissue rebuilding parameters such as signaling by heparin-binding growth factors (FGF-2, VEGF) and collagen processing in fibroblasts, smooth muscle cells or endothelial cells in culture. LMWF can bind fibrillar collagens and provide protection and signal promotion of heparin binding growth factors to improve biocompatibility of purified cancellous bone substitute. Indeed, it was demonstrated that LMWF mimicks and restores the properties of bone non collagenous matrix (proteoglycans, glycoproteins)
that were eliminated by drastic purification process during design of the biomaterial, to regulate soluble factors bioavailability [29].
In recent years, fucoidan or FCSPs from seaweed has many scien- tific studies aiming at assessing their potential biological functions including antitumor and immunomodulatory [30–32], antivirus [33], antithrombotic and anticoagulant [34], anti-inflammatory [35], and antioxidant effects [36], as well as their effects against various renal [37], hepatic [38] and uropathic disorders [39]. The bioactivity of fucoidan was isolated from brown seaweed such as Undaria and Laminaria showed anticoagulant, antiviral and anticancer properties [40,41]. Fucoidan is an excellent nat- ural antioxidant has significant antioxidant activity. Sulphated polysaccharides from the marine algae Porphyra haitanesis [42], Ulva pertusa [43], F. vesiculosus [44], Laminaria japonica [45] and Ecklonia kurome [46] has demonstrated to possess as antioxidant activity. Fucoidan was extracted from L. japonica, a commercially important algae species in China. Three sulphated polysaccharide fractions were successfully isolated through anion exchange col- umn chromatography and their antioxidant activities investigated employing various established in vitro systems, including superox- ide and hydroxyl radical scavenging activity, chelating ability and reducing power [47]. Fucoidan stimulates the immune system in several ways, and the numerous important biological effects are related to their ability to modify cell surface properties [48]. Oral intake of the fucoidans present in dietary brown seaweed might take the protective effects through direct inhibition of viral repli- cation and stimulation of the immune system (innate and adaptive) functions [49]. The mechanism of antiviral activities of fucoidan is to inhibit viral sorption so as to inhibit viral-induced syncytium formation. Sulphate is necessary for the antiviral activity, and of (1–3)-linked fucopyranosyl units appears to be very important for the anti-herpetic activity of fucoidan [50].
The venous antithrombotic activity of LMW fucans (LMWF) has compared with a low-molecular-weight heparin in the Wessler rabbit model and exhibited a better ratio antithrombotic effect/hemorrhagic risk [1,51,52]. This antithrombotic activity may, in part, be explained by the decrease of tissue factor expression in the media of denuded arteries and the significant increase of plasma TFPI (tissue factor pathway inhibitor) released from endothelial cell [53,54]. Vasculogenesis is the process of blood vessel for- mation occurring by a production of endothelial cells. Fucoidan induces endothelial progenitor cell (EPCs) proliferation, migration and differentiation into capillary-like structures on Matrigel [55]. Low molecular weight fucoidan (LMWF) could act through stromal
cell-derived factor (SDF-1), which when stimulated EPCs in hind limb ischemia [56,57]. LMWF can also promote tissue rebuilding parameters such as signaling by heparin-binding growth factors (FGF-2, VEGF) and collagen processing in fibroblasts, smooth mus- cle cells or endothelial cells in culture. LMWF can bind fibrillar collagens and provide protection and signal promotion of heparin binding growth factors to improve biocompatibility of purified can- cellous bone substitute. Indeed, it was demonstrated that LMWF mimics and restores the properties of bone non collagenous matrix (proteoglycans, glycoproteins) that were eliminated by drastic purification process during design of the biomaterial, to regulate soluble factors bioavailability [29].
A large molecular weight is required to achieve anticoagulant activity as fucoidan needs a long sugar chain in order to be able to bind the thrombin (coagulation protein in the blood stream). Some researchers have measured fucoidan’s molecular weight at approximately 100 kDa, even as others have observed a molecular weight of 1600 kDa [58]. Heparin is a biomolecule containing highly sulfated glucosaminoglycan that is widely used as an injectable anticoagulant. It has reported that the anticoagulant mechanisms of fucoidan are related to both antithrombin and heparin cofactor II-mediated activity [59]. The structural and anionic characteristics of fucoidan are similar to those of heparin. Heparin stimulates pro- duction of hepatocyte growth factor (HGF), which has key roles in tissue regeneration. Fucoidan and fucoidan-derived oligosaccha- rides have similar ability to stimulate production of hepatocyte growth factor (HGF). This induction of HGF by heparin or fucoidan and their oligosaccharide derivates occurs primarily at the level of translation. Thus, fucoidan may be useful to protect tissues and organs from various injuries and diseases, via mechanisms involv- ing HGF [60].
Fucoidan from L. japonica reduced serum total and LDL- cholesterol and triglycerides and raised HDL-cholesterol in a hyperlipidemic rat model [61]. Some studies suggest that fucoidan has potential for use as an anti-inflammatory agent. Fucoidan treatment led to less severe symptoms in the early stages of Staphy- lococcus aureus triggered arthritis in mice, but delayed phagocyte recruitment and decreased clearance of the bacterium [62]. Also, injection of fucoidan into sensitized mice with before hapten challenge reduced the contact hypersensitivity reactions [63]. Fur- thermore, recruitment of leukocytes into cerebrospinal fluid in a meningitis model is reduced by fucoidan [64], as is IL-1 produc- tion in a similar model [65]. Also, fucoidan has studied in bone tissue engineering. Recent studies evidenced that hydroxyapatite- fucoidan (HApF) nanocomposite for bone tissue engineering may be promising biomaterial and could be used for bone tissue con- struct [66].
Cancer is a group of diseases characterized by uncontrolled growth and spread of abnormal cells. If the spread is not con- trolled, it can result in death. Despite considerable progress in medical research, cancer remains one of the high-ranking causes of death in the world. The National Cancer Institute estimates that “approximately 11.4 million Americans with a history of cancer were alive in January 2006. In 2012, about 577,190 Americans are expected to die of cancer, more than 1500 people a day. Cancer is the second most common cause of death in the US, exceeded only by heart disease, accounting for nearly 1 of every 4 deaths (Source: Cancer Facts and Figs. 2012 of the American Cancer Soci- ety). Accordingly, research must continue to progress to improve existing therapies and to develop novel cures. For many years, research has essentially focused on plants and terrestrial microor- ganisms, mainly because of these specimens are easily available and folk traditions have described beneficial effects from their use. Several different therapeutic strategies such as chemother- apy, radiation therapy, surgery or combinations have been used to treat different types of cancer. Unfortunately, several of these treat- ments provide only minimal benefits; moreover, complications and long term side effects of these treatments [67,68]. The therapeutic potential of natural bioactive compounds such as polysaccharides, especially fucoidan is now well documented, and this activity com- bined with natural biodiversity will allow the development of a new generation of therapeutic measures against cancer has been going on for years and recently the focus has been directed toward bioactive compounds of natural origin, including FCSPs from brown seaweeds [10].
Fucoidan is known to have anti-tumor effects, but its mode of action is not fully understood. Some of the marine brown seaweed fucoidan on cancer target has listed in Table 1. Fucoidan found to inhibits proliferation and induce apoptosis in human lymphoma HS-Sultan cell lines [18]. Fucoidans from brown seaweeds Eclonia cava, Sargassum hornery, and Costaria costata showed that anti- cancer effect on human melanoma and colon cancer cells [69]. Human malignant melanoma cancer cell (SK-MEL-28 and SK-MEL- 5) growth was inhibited by native fucoidan was isolated from Fucus evanescens [70]. Fucoidans from L. saccharina, L. digitata, F. serra- tus, F. distichus and F. vesiculosus strongly blocked MDA-MB-231 breast carcinoma cell adhesion and implications in tumor metasta- sis [71]. Alekseyenko et al. studied the antitumor and antimetastatic activities of fucoidan from Fucus evanescens in C57Bl/6 mice with transplanted Lewis lung adenocarcinoma. Fucoidan after single and repeated administration in a dose of 10 mg/kg produced moder- ate antitumor and antimetastatic effects [30]. The animals were fed with a diet containing 1% fucoidan from Mekabu for 10 days and subcutaneously (s.c.) inoculated with A20 leukemia cells. Thereafter, the mice were fed with the diet containing fucoidan for 40 days. Mekabu fucoidan inhibited tumors by 65.4% [31]. Native and oversulfated FCSPs derived from Cladosiphon okamuranus (Chordariales) was analyzed using 1H NMR spec- troscopy and sulfation produced 4-mono-O-sulfo-l-fucopyranose the oversulfated FCSPs contained 2,4-di-, 2-mono-, and 4-mono- O-sulfo-l-fucopyranose that sulfate content and the positioning of sulfate groups, e.g., 2,4-di- vs. 4-mono, might be important for the anti-proliferative activity of fucoidan in a human leukemia cell line (U937) [72]. Sulfated polysaccharides from brown seaweeds S. japonica and U. pinnatifida possessed high antitumor activity and inhibit proliferation and colony formation of breast cancer and melanoma cell lines [73] (Table 2).
Fucoidan on cell cycle and apoptosis
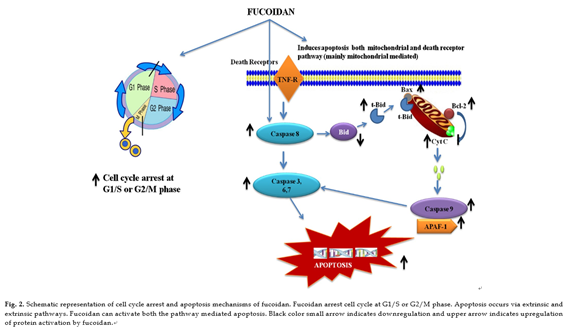
The fundamental processes of progression through the cell cycle and of programmed cell death involve the complex interaction of several families of proteins in a systematic and coordinated man- ner. They are separate, distinct processes that are intimately related and together play an important role in the sensitivity of malig- nant cells to chemotherapy. The cell cycle is the mechanism by which cells divide. It is a high energy demanding process that requires an encompassed and ordered series of events to guarantee the correct duplication and segregation of the genome. This pro- cess involves four sequential phases that go from quiescence (G0 phase) to proliferation (G1, S, G2, and M phases) and back to qui- escence [74]. Increasing knowledge on the cell cycle deregulations in cancers has promoted the introduction of marine bioactive com- pounds, which can either modulate signaling pathways leading to cell cycle regulation or directly alter cell cycle regulatory molecules, in cancer therapy. Fucoidan showed that at 1.0 mg/ml concentra- tion increased the G0/G1-phase population in hepatocarcinoma cell line (Huh7) accompanying by a decrease in the S phase, suggesting that fucoidan may cause the cell cycle arrest at the G0/G1 phase [75]. Fucoidan suppressed cell proliferation and arrest cell cycle in HCC cell lines (HAK-1A, KYN-2, KYN-3) revealed an increased number of cells in the G2/M phase at 72 h after the addition of the fucoidan (22.5 µg/ml) [76]. Fucoidan induced the accumula- tion of cells in G1/S phase of the cell cycle on HUT-102 cells (T-cell lymphoma) [22] and non-small-cell human bronchopulmonary carcinoma (NSCLC-N6) cells [19]. The growth-inhibitory function of fucoidan on human T-cell leukemia virus type 1 (HTLV-1) infected T cells was mainly due to the induction of cell cycle arrest and apo- ptosis because a significant population of cells remained in the G1 phase of cell cycle and underwent apoptosis after treatment with fucoidan. Fucoidan-induced G1 phase accumulation was accompa- nied by downregulation of cyclin D2 and also downregulation of c-myc and pRb phosphorylation in HTLV-1-infected T-cell line [22]. Apoptosis is critically important for the survival of multicellular organisms [77]. The process of programmed cell death, or apoptosis,
is generally characterized by distinct morphological characteristics and energy-dependent biochemical mechanisms. Impairment of this native defense mechanism promotes aberrant cellular prolifer- ation and the accumulation of genetic defects, ultimately resulting in tumorigenesis [78]. Fucoidan suppressed cell proliferation in a time- and dose-dependent manner, and its effects were particularly pronounced in the HCC, cholangiocarcinoma and gallbladder car- cinoma cell lines indicated that the mechanisms of fucoidan action include the induction of apoptosis and the inhibition of the cell cycle [76].
Apoptosis is mediated through two major pathways, the extrinsic (death receptor) and intrinsic (mitochondrial) pathways represent the two major well-studied apoptotic processes [79,80]. In the extrinsic pathway, stimulation of death receptors, such as Fas and tumor necrosis factor receptor-1, leads to clustering and formation of a death-inducing signaling complex, which includes the adaptor protein Fas-associated death domain (FADD) and ini- tiator caspases, such as caspase-8. Activated caspase-8 directly activates downstream effector caspases, such as caspase-3 and -7 [81]. Also, caspase-8 can cleave Bid (Bcl-2 interacting protein) into tBid (truncated Bid), and interacts with proapoptotic protein Bax, and the accumulation of Bax in mitochondria promotes cytochrome c released into the cytosol [82–84]. In the intrinsic pathway, death receptors transmit death signals to the mitochondria, resulting in the release of several mitochondrial intermembrane space proteins, such as cytochrome c, which associate with Apaf-1 and procaspase- 9 to form the apoptosome. Activated caspase-9 can cleave and activate effector caspases, such as caspase-3 and -7 [85]. Stud- ies showed that fucoidan, extracted from Cladosiphon okamuranus, strongly antiproliferative and apoptotic effects on MCF-7 cells in a dose-dependent manner. However, fucoidan did not affect pro- liferation of normal cells of human mammalian epithelial (HMEC) cells. The characteristics of apoptotic cell death are induction of chromatin condensation, fragmentation of nuclei and DNA, and cleavage of specific proteins. Fucoidan induced accumulation of sub-G1 population, chromatin condensation, and internucleosomal fragmentation of DNA [86]. Because these are representative fea- tures of apoptosis, fucoidan was shown to induce apoptotic cell death in MCF-7 cells. Effector caspases, such as caspase-3 or -7, activate DNase, resulting in fragmentation of DNA in response to various apoptotic stimuli. MCF-7 cells show a defect in caspase-3 but express caspase-7, which is an executioner caspase capable of cleaving PARP (Poly (ADP-ribose) polymerase) [87]. Activation of caspase-7 and PARP cleavage are hallmarks of apoptosis in MCF- 7 cells [88]. Cleavage of PARP and activation of caspase-7 were induced after treatment with fucoidan in MCF-7 cells and that caspase-7 inhibitor z-DEVD-fmk canceled fucoidan-induced apo- ptosis. Caspase-3 is known to be activated and plays a pivotal role in fucoidan-induced cell death [18,72]. Taken together, caspase- 7 is required by MCF-7 cells and that activation of caspase-3 is not necessarily a prerequisite for fucoidan-induced apoptosis [86]. Fucoidan (Fucoidan extract) increased mitochondrial depolariza- tion by up-regulates the expression of pro-apoptotic proteins Bax and Bad, and down-regulates the expression of anti-apoptotic pro- teins Bcl-2 and Bcl-xl in MCF-7 cells [24].
Caspase-8 plays a crucial role in apoptosis triggered by the inter- action of ligand with death receptors, such as Fas, tumor necrosis factor receptor (TNFR), and TNF-related apoptosis inducing ligand receptor (TRAIL-R). Bid is directly cleaved by caspase-8, where- upon the C-terminal BH3 domain containing a fragment of Bid called as tBid, could triggers cytochrome c release and leading to caspase-9 activation. Fucoidan activate Bid into tBid in MCF-7 cells. Therefore, it is also suggested that caspase-8 activates the tBid-related apoptotic pathway, leading to caspase-9 activation as well as direct activation of capase-7 in fucoidan-treated cells. The activated caspase-9 then proteolytically cleaves and activates
executioner caspase-3, -6, and -7 [89]. Activation of caspase-9 appears to be a branched pathway of apoptotic cell death, origi- nating from caspase-8 during fucoidan-induced apoptosis.
The F. vesiculosus FCSPs treatment was also shown to enhance mitochondrial membrane permeability of human colon cancer cells in vitro, and to induce cytochrome c and Smac/Diablo release from the mitochondria [90]. Studies from Jin et al. [91] reported that the intracellular levels of apoptotic proteins modulated in fucoidan-treated HL-60 cells. The levels of the active forms of caspases-8, caspase-9, and caspase-3 increased in response to fucoidan in a dose-dependent manner and also the cleavage of PARP, a typical substrate for caspase-3. Moreover, the anti- apoptotic protein, Bid, decreased in a time-dependent fashion in response to treatment with fucoidan. Haneji et al. [22] reported that apoptosis is induced by the activation of the caspase pathway, and Aisa et al. [18] demonstrated anti-tumor effects accompanied by the activation of the caspase pathway and the down-regulation of the ERK pathway. LMWF induces a caspase independent, mitochondrial-mediated apoptotic pathway in ER-positive MCF-7 cells [24].
Zhang et al. [26] showed that LMWF induces a sustained col- lapse of mitochondrial membrane potency, release of cytochrome c, and down regulation of antiapoptotic proteins Bcl-2, Mcl-1, Bcl-xl, and activation of caspase-9, caspase-7, caspase-3, thereby induces apoptosis in MDA-MB-231 cells. Another way changes in Ca2+ signaling can affect cell proliferation and differentia- tion and modulate apoptosis. LMWF evoked a rapid increase in the intracellular Ca2+ level in MDA-MB-231 cells, and this
increase was significantly inhibited by the Ca2+ chelator, BAPTA- AM [1,2-Bis(2-aminophenoxy)ethane-N,N,Nr,Nr-tetraacetic acid tetrakis (acetoxymethyl ester)] suggested that the disruption of Ca2+ homeostasis played a role in the apoptotic process [26]. Cal-
pain is a protein belonging to the family of calcium-dependent, non-lysosomal cysteine proteases present in the cytosol as the inactive proenzyme, procalpain, which translocates to the cell membrane in the presence of Ca2+. One important substrate for calpain is α-spectrin (or fodrin) has implicated as a death sub- starte, which cleaved and play a role in regulating membrane structure, cell shape and linking the cytoskeleton to the plasma membrane or intracellular vehicles [92]. The detection of calpain induced α-spectrin proteolytic fragments has great importance between calpain activation and the earliest stage of apoptosis. LMWF degraded α-spectrin into 150 kDa and 120 kDa fragments. The E-64d calpain inhibitor reduced the production of the 150 kDa fragment, these findings suggests that both calpain and caspases
participated in LMWF induced apoptosis [26].
Studies from Ale et al. [32] proposed mechanism for inhibition of proliferation and induce apoptosis of melanoma cells by FCSPs that activation of macrophages via membrane receptors, which leads to the production of cytokines that enhance natural killer (NK) cell activation. Activated NK cells release Granzyme B and perforin through granule exocytosis into the space between NK cells and melanoma cells to initiate caspase cascades in melanoma cells. Granzyme B then initiates apoptosis by triggering the release of mitochondrial cytochrome c and apoptosome formation lead- ing to caspase-3 activation, which in turn translocates the nucleus causing DNA fragmentation the distinct morphological change of cells by apoptosis [32]. The antitumor activity of fucoidan from Fucus vesiculosus was investigated in human colon carcinoma cells. The crude fucoidan, composed predominantly of sulfated fucose, markedly inhibited the growth of HCT-15 cells (human colon carci- noma cells) by several apoptotic events such as DNA fragmentation, chromatin condensation and increase in the population of sub-G1 cells and mediates apoptosis signaling through mitochondrial path- way [93]. The fucoidan regulated mitochondrial and death receptor apoptotic signaling molecules is shown in Fig. 2.
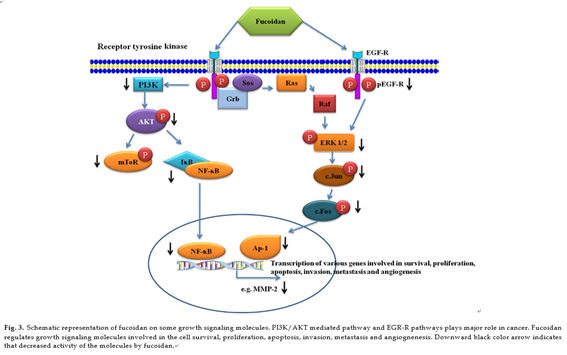
Published data indicate that various compounds exert chemo- preventive and chemotherapeutic effects through the inhibition of phosphorylation of membrane receptors, including recep- tor tyrosine kinases (RTKs), EGFR and platelet-derived growth factor receptor (PDGFR), are involved in the transduction of mitogenic signals across the plasma membrane and in the regulation of cell growth and proliferation. Some marine com- pounds efficiently interrupt constitutive growth factor stimulated cell signaling pathways, typically triggering a pathway involv- ing Ras Raf extracellular regulated kinase (ERK) mitogen activated kinase/ERK-kinase (MEK) activator protein (AP)-1 pathway. Mitogen activated protein kinase (MAPK) pathways are involved in cellular proliferation, differentiation, and apoptosis [94,95]. ERK1/2 pathway is involved in the invasive or migratory behavior of a number of malignancies [96–98]. Anti-metastatic effect of fucoidan is owing to its inactivation of ERK1/2 path- way in A549 human lung cancer cells. In addition to ERK1/2, the PI3K–Akt signal pathway has been shown to regulate the invasion and metastasis of non small-cell lung cancer (NSCLC) as well as the development and progress of various other tumors. PI3K overex- pression is highly correlated with the development, invasion, and metastasis of NSCLC [99]. Fucoidan inhibits the phosphorylation of PI3K–Akt in time and concentration-dependent manners. Mam- malian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth and proliferation, cell motility, cell survival, protein synthesis, and transcription. PI3K and Akt are well known upstream regulators of mTOR signaling pathway in mammalian cells. Fucoidan effectively down regulates the expres- sion of MMP-2 through the inhibitions of PI3K–Akt–mTOR as well as ERK1/2 signaling pathways in A549 human lung cancer cells. Fucoidan significantly inhibited phospho-ERK1/2, p-PI3K, p-AKt in a concentration-dependent manner with a maximum inhibitory effect at the highest concentration (200 µg/ml). Additionally, 4E- BP1 and p70S6K, two immediate downstream targets of mTOR
and indicators of mTOR activity, were also significantly suppressed [100]. NF-nB and AP-1 are transcription factors that regulate the expressions of numerous genes associated with many important biological and pathological processes, including cancer and it has reported that inhibition of NF-nB and AP-1 results in the sup- pression of tumor initiation, promotion and metastasis [101,102]. Fucoidan inhibits the phosphorylation of In-Bα and increased the total p65 in the cytosolic fraction and its decreased in nuclear frac- tion in lung cancer cells A549 [100].
Fucoidan exerted a potent inhibitory effect on EGF-induced phosphorylation of EGFR. Subsequently, fucoidan suppressed the phosphorylation of ERK and JNK under the control of EGF. Interest- ingly, EGF-induced c-fos and c-jun transcriptional activities were inhibited by fucoidan, leading to inhibition of activator protein- 1 (AP-1) activity and cell transformation induced by EGF [103]. AP-1 is a transcription factor involved in cellular proliferation and transformation and is transactivated by various tumor-promoting agents such as phorbol ester and ultraviolet irradiation [104]. AP-1 complexes are formed by dimmers of Jun proto-oncogenes or het- erodimers of the Jun family members with the Fos proto-oncogene family members. AP-1 binds to a specific target DNA site in the pro- moters of several cellular genes and mediates immediate early gene expression involved in a diverse set of transcriptional regulation processes [105]. Fucoidan suppress the AP-1 activation through inhibition of JunD expression in an HTLV-1-infected T-cell line, thereby inhibits HTLV-1-infected T-cell proliferation [22]. The reg- ulation growth signal molecules by fucoidan is shown in Fig. 3.
Metastasis is a leading cause (up to 90%) of cancer-related deaths. The development of cancer metastasis consists of multi- ple processes, in which cancer cells first detach from the primary tumor, invade surrounding tissues and intravasate into blood and/or lymphatic systems and extravasate from the vasculature and subsequently settle and colonize at the target organs. Matrix
metalloproteinases (MMPs) play a key role in tumor metastasis. The degradation of extracellular matrix (ECM) is crucial for cel- lular invasion, indicating the inevitable involvement of matrix degrading proteinases for the process. Fucoidan suppressed MMP- 2 activity and protein expression with increasing concentrations of fucoidan in A549 (lung) cancer cells [100]. Fucoidan demonstrated that inhibit metastasis via MMP-2 suppression as well as subdue the expression and secretion of a vascular endothelial growth fac- tor (VEGF) [25]. The anti-metastatic activity of fucoidan was also proven in the animal model of experimental transplanted Lewis lung carcinoma (LLC) cells [30]. Fucoidan was shown to influence the function of several cell surface proteins involved in migration and cell adhesion, including integrins, VEGF 1 and 2, P-selectin and neuropilin-1 [106–108].
Angiogenesis is the new blood vessels formation in physiological and pathological processes [109,110]. Highly regulated and tran- sient angiogenesis is necessary for embryonic development and wound healing. However, uncontrolled and persistent angiogenesis occurs in several pathological states including tumor progression. Tumor growth is requires for angiogenesis to supply nutrients and oxygen. Anti-angiogenic therapy has become an effective strat- egy for inhibiting tumor growth. Inhibition of angiogenesis can leads to target for cancer therapy [111]. Oversulfated fucoidan enhances anti tumor and anti angiogenic effects on cancer [112]. Fucoidan and oversulfated fucoidan significantly suppressed the mitogeneic and chemotactic actions of VEGF. Vascular endothe- lial growth factor (VEGF) is an interesting inducer of angiogenesis and lymphangiogenesis, because it is a highly specific mitogen for endothelial cells. SDF-1 (stromal cell-derived factor-1) is a small cytokine belonging to the chemokine family that designated as Chemokine (C-X-C motif) ligand 12 (CXCL12). The receptor for this chemokine is CXCR4. Targeting the CXCL12/CXCR4 pathway is a
logic strategy in cancer therapy [113,114]. Fucoidan inhibited cell growth more prominently in Huh7 human hepato cancer cells by suppressing chemotaxin CXCL12 and CXCR4 [75]. Both native and oversulfated FCSPs have anti-angiogenic actions in vivo and in vitro anti-proliferative effects against B16 melanoma cells, Sarcoma-180 and Lewis lung carcinoma cells. The interaction of oversulfated FCSPs with VEGF165 occurred with high affinity and resulted in the formation of highly stable complexes, thereby interfering with the binding of VEGF165 to vascular endothelial growth factor receptor- 2 (VEGFR-2). The study showed that both native and oversulfated FCSPs were able to suppress neovascularization in mice implanted Sarcoma-180 cells; and that both FCSPs types inhibited tumor growth through the prevention of tumor-induced angiogenesis, but the data indicated that sulfation tended to give more potent effects [112]. Fucoidan inhibited both human glioblastoma (T98G) and THP1 (acute monocytic leukemia) cell-induced angiogenesis [115].
Using a human umbilical vein endothelial cell (HUVEC)- based cell culture model, the anti-angiogenic activity of fucoidan extracted from the brown seaweed Undaria pinnatifida showed significant inhibition of cell proliferation, cell migration, tube for- mation and vascular network formation. Also ex vivo angiogenesis assay demonstrated that 100 µg/ml of fucoidan caused significant reduction in microvessel outgrowth. Western blot and RT-PCR anal- yses indicated that at 400 µg/ml, fucoidan significantly reduced the expression of the angiogenesis factor VEGF-A in the suppres- sion of angiogenesis activity [116]. Recent studies also showed that fucoidan, significantly reduces tumor volume and the number of metastatic lung nodules in the 4T1 xenograft model. The mecha- nisms involved in the reduction by fucoidan of tumor growth in 4T1-bearing mice, demonstrated that fucoidan suppresses in vitro cell proliferation, colony formation, expression of epithelial to mesenchymal transition (EMT) biomarkers and blocks cell migra- tion and cell invasion [117]. The molecular network of transforming growth factor β (TGF-β) receptors (TGFRs) plays an important role in the regulation of the EMT in cancer cells. Using 4T1 and MDA- MB-231 cells, fucoidan effectively reverses TGF-R induced EMT morphological changes, upregulates epithelial markers, downreg- ulates mesenchymal markers and decreases the expression of transcriptional repressors Snail, Slug and Twist. Fucoidan decreases TGF-RI and TGF-RII proteins and affects downstream signaling molecules, including Smad2/3 phosphorylation and Smad4 expres- sion. The study was shown to identify a novel mechanism for fucoidan antitumor activity, namely regulation of the EMT via modulation of TGFR/Smad-dependent signaling, which leads to an inhibition of breast cancer cell growth in vitro and in vivo. The findings indicated that fucoidan is a potential therapeutic agent for breast cancer and acts via an ubiquitin-dependent degradation pathway that affects the TGFR/Smad/Snail, Slug, Twist and EMT axes [117].
6. Conclusions
Seaweeds are a group of marine multicellular algae has various health benefits and biomedical applications. Brown seaweeds con- tains fucoidans are complex and heterogeneous, and have various structures, but not been very clear until now. Fucoidan has various biological activities which include antitumor, immunomodulatory, antiviral, antithrombotic, anticoagulant, antithrombotic, antiox- idant and antilipidemic activity. Fucoidan inhibits cancer cell proliferations by inducing cell cycle arrest, inducing apoptosis, regulating growth signaling molecules and inhibits metastasis and angiogenesis. Deeply studying the structure of fucoidans and exploring the relationship activity and structure are useful for developing and utilizing the brown algae resource. Future studies also develop with combinations of fucoidan or LMWF with other anticancer agents could be very good target for anticancer therapy.
This study was supported by a grant from Marine Bioprocess Research Center of the Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Republic of Korea.
Costantino, E. Fattorusso, M. Menna, O. Taglialatela-Scafati, Current Medic- inal Chemistry 11 (2004) 1671–1692.
K. Chandini, P. Ganesan, P. Suresh, N. Bhaskar, Journal of Food Science and Technology 45 (2008) 1–13.
L. Holdt, S. Kraan, Journal of Applied Phycology 23 (2011) 543–597.
Bhadury, B.T. Mohammad, P.C. Wright, Journal of Industrial Microbiology and Biotechnology 33 (2006) 325–337.
Murata, J.-i. Nakazoe, Japan Agricultural Research Quarterly 35 (2001) 281–290.
J. McHugh, Production and utilization of products from commercial sea- weeds, FAO Fisheries Technical Paper 288, 1987, pp. 58–115.
Tseng, Journal of Applied Phycology 13 (2001) 375–380.
Jensen, Component sugars of some common brown algae, Akademisk Trykningssentral, Blindern, Oslo. Report 9, Norwegian Institute of Seaweed Research, 1956.
[9] S. Patel, 3 Biotech 2 (2012) 1–15.
Jiao, G. Yu, J. Zhang, H.S. Ewart, Marine Drugs 9 (2011) 196–223.
Kylin, Hoppe-Seyler’ s Zeitschrift für physiologische Chemie 83 (1913) 171–197.
Berteau, B. Mulloy, Glycobiology 13 (2003) 29–40.
Percival, R.H. McDowell, Chemistry and Enzymology of Marine Algal Polysaccharides, Academic Press, London, 1967, pp. 219.
S. Patankar, S. Oehninger, T. Barnett, R. Williams, G. Clark, Journal of Bio- logical Chemistry 268 (1993) 21770–21776.
I. Bilan, A.I. Usov, ChemInform 40 (2009) 34.
E. Duarte, M.A. Cardoso, M.D. Noseda, A.S. Cerezo, Carbohydrate Research 333 (2001) 281–293.
Tako, E. Yoza, S. Tohma, Botanica Marina 43 (2000) 393–398.
Aisa, Y. Miyakawa, T. Nakazato, H. Shibata, K. Saito, Y. Ikeda, M. Kizaki, American Journal of Hematology 78 (2005) 7–14.
Riou, S. Colliec-Jouault, d.S.D. Pinczon, S. Bosch, S. Siavoshian, V. Le Bert, C. Tomasoni, C. Sinquin, P. Durand, C. Roussakis, Anticancer Research 16 (1996) 1213–1218.
Yamamoto, M. Takahashi, T. Suzuki, H. Seino, H. Mori, Japanese Journal of Experimental Medicine 54 (1984) 143–151.
Itoh, H. Noda, H. Amano, H. Ito, Anticancer Research 15 (1995) 1937–1947.
Haneji, T. Matsuda, M. Tomita, H. Kawakami, K. Ohshiro, J.N. Uchihara,
Masuda, N. Takasu, Y. Tanaka, T. Ohta, Nutrition and Cancer 52 (2005) 189–201.
Matsubara, C. Xue, X. Zhao, M. Mori, T. Sugawara, T. Hirata, International Journal of Molecular Medicine 15 (2005) 695–700.
Zhang, K. Teruya, H. Eto, S. Shirahata, PLoS ONE 6 (2011) 1–14.
Ye, Y. Li, K. Teruya, Y. Katakura, A. Ichikawa, H. Eto, M. Hosoi, M. Hosoi, S. Nishimoto, S. Shirahata, Cytotechnology 47 (2005) 117–126.
Zhang, K. Teruya, H. Eto, S. Shirahata, Bioscience, Biotechnology, and Bio- chemistry 77 (2013) 235–242.
Haroun-Bouhedja, M. Ellouali, C. Sinquin, C. Boisson-Vidal, Thrombosis Research 100 (2000) 453–459.
Senni, F. Gueniche, A. Foucault-Bertaud, S. Igondjo-Tchen, F. Fioretti, S. Colliec-Jouault, P. Durand, J. Guezennec, G. Godeau, D. Letourneur, Archives of Biochemistry and Biophysics 445 (2006) 56–64.
Changotade, G. Korb, J. Bassil, B. Barroukh, C. Willig, S. Colliec Jouault, P. Durand, G. Godeau, K. Senni, Journal of Biomedical Materials Research A 87 (2008) 666–675.
Alekseyenko, S.Y. Zhanayeva, A. Venediktova, T. Zvyagintseva, T. Kuznetsova, N. Besednova, T. Korolenko, Bulletin of Experimental Biology and Medicine 143 (2007) 730–732.
Maruyama, H. Tamauchi, M. Iizuka, T. Nakano, Planta Medica 72 (2006) 1415–1417.
T. Ale, H. Maruyama, H. Tamauchi, J.D. Mikkelsen, A.S. Meyer, International Journal of Biological Macromolecules 49 (2011) 331–336.
Makarenkova, P. Deriabin, D. L‘vov, T. Zviagintseva, N. Besednova, Voprosy Virusologii 55 (2010) 41–45.
Zhu, Q. Zhang, L. Chen, S. Ren, P. Xu, Y. Tang, D. Luo, Thrombosis Research 125 (2010) 419–426.
Semenov, A. Mazurov, M. Preobrazhenskaia, N. Ushakova, V. Mikha˘ılov,
Berman, A. Usov, N. Nifant’ev, N. Bovin, Voprosy medit sinsko˘ı khimii 44 (1998) 135–140.
Wang, Q. Zhang, Z. Zhang, H. Song, P. Li, International Journal of Biological Macromolecules 46 (2010) 6–12.
K. Veena, A. Josephine, S.P. Preetha, P. Varalakshmi, R. Sundarapandiyan, Life Sciences 79 (2006) 1789–1795.
Hayakawa, T. Nagamine, Anticancer Research 29 (2009) 1211–1217.
Zhang, N. Li, T. Zhao, H. Qi, Z. Xu, Z. Li, Phytotherapy Research 19 (2005) 50–53.
Chevolot, B. Mulloy, J. Ratiskol, A. Foucault, S. Colliec-Jouault, Carbohydrate Research 330 (2001) 529–535.
Zhuang, H. Itoh, T. Mizuno, H. Ito, Bioscience, Biotechnology, and Biochem- istry 59 (1995) 563–567.
Zhang, N. Li, G. Zhou, X. Lu, Z. Xu, Z. Li, Pharmacological Research: The Official Journal of the Italian Pharmacological Society 48 (2003) 151.
Qi, T. Zhao, Q. Zhang, Z. Li, Z. Zhao, R. Xing, Journal of Applied Phycology 17 (2005) 527–534.
Rupérez, O. Ahrazem, J.A. Leal, Journal of Agricultural and Food Chemistry 50 (2002) 840–845.
Xue, L. Chen, Z. Li, Y. Cai, H. Lin, Y. Fang, Developments in Food Science 42 (2004) 139–145.
F. Hu, M.Y. Geng, J.T. Zhang, H.D. Jiang, Journal of Asian Natural Products Research 3 (2001) 353–358.
Wang, Q. Zhang, Z. Zhang, Z. Li, International Journal of Biological Macro- molecules 42 (2008) 127–132.
Usov, G. Smirnova, N. Klochkova, Russian Journal of Bioorganic Chemistry 27 (2001) 395–399.
Hayashi, N.S. Yokoya, S. Ostini, R.T. Pereira, E.S. Braga, E.C. Oliveira, Aqua- culture 277 (2008) 185–191.
Mandal, C.G. Mateu, K. Chattopadhyay, C.A. Pujol, E.B. Damonte, B. Ray, Antiviral Chemistry & Chemotherapy 18 (2007) 153–162.
Colliec-Jouault, J. Millet, D. Helley, C. Sinquin, A. Fischer, Journal of Throm- bosis and Haemostasis 1 (2003) 1114–1115.
Millet, S.C. Jouault, S. Mauray, J. Theveniaux, C. Sternberg, C.B. Vidal, A. Fischer, Journal of Thrombosis and Haemostasis 81 (1999) 391–395.
Durand, D. Helley, A. Al Haj Zen, C. Dujols, P. Bruneval, S. Colliec-Jouault, A.-M. Fischer, A. Lafont, Journal of Vascular Research 45 (2008) 529–537.
L. Giraux, J. Tapon-Bretaudière, S. Matou, A.M. Fischer, Journal of Thrombosis and Haemostasis 80 (1998) 692–695.
Zemani, D. Benisvy, I. Galy-Fauroux, A. Lokajczyk, S. Colliec-Jouault, G. Uzan, A.M. Fischer, C. Boisson-Vidal, Biochemical Pharmacology 70 (2005) 1167–1175.
A. Sweeney, G.V. Priestley, B. Nakamoto, R.G. Collins, A.L. Beaudet, T. Papayannopoulou, Proceedings of the National Academy of Sciences of the United States of America 97 (2000) 6544–6549.
Zemani, J.S. Silvestre, F. Fauvel-Lafeve, A. Bruel, J. Vilar, I. Bieche, I. Lau- rendeau, I. Galy-Fauroux, A.M. Fischer, C. Boisson-Vidal, Arteriosclerosis, Thrombosis, and Vascular Biology 28 (2008) 644–650.
E. Rioux, S. Turgeon, M. Beaulieu, Carbohydrate Polymers 69 (2007) 530–537.
Nishino, T. Nagumo, Carbohydrate Research 214 (1991) 193–197.
Fukuta, T. Nakamura, Journal of Pharmacy and Pharmacology 60 (2008) 499–503.
Huang, K. Wen, X. Gao, Y. Liu, Pharmaceutical Biology 48 (2010) 422–426.
Verdrengh, H. Erlandsson-Harris, A. Tarkowski, European Journal of Immunology 30 (2000) 1606–1613.
Nasu, Y. Fukuda, K. Nagahira, H. Kawashima, C. Noguchi, T. Nakanishi, Immunology Letters 59 (1997) 47–51.
Granert, J. Raud, A. Waage, L. Lindquist, Infection and Immunity 67 (1999) 2071–2074.
Ostergaard, R. Yieng-Kow, T. Benfield, N. Frimodt-Møller, F. Espersen, J. Lundgren, Infection and Immunity 68 (2000) 3153–3157.
S. Jeong, J. Venkatesan, S.K. Kim, International Journal of Biological Macro- molecules 57 (2013) 138–141.
Schneider, A. Stipper, J. Besserer, Zeitschrift für Medizinische Physik 20 (2010) 206–214.
Grossi, K. Kubota, F. Cappuzzo, F. de Marinis, C. Gridelli, M. Aita, J.Y. Douil- lard, Oncologist 15 (2010) 1102–1112.
Ermakova, R. Sokolova, S.-M. Kim, B.-H. Um, V. Isakov, T. Zvyagintseva, Applied Biochemistry and Biotechnology 164 (2011) 841–850.
D. Anastyuk, T.I. Imbs, N.M. Shevchenko, P.S. Dmitrenok, T.N. Zvyagintseva, Carbohydrate Polymers 90 (2012) 993–1002.
Cumashi, N.A. Ushakova, M.E. Preobrazhenskaya, A. D‘Incecco, A. Piccoli,
Totani, N. Tinari, G.E. Morozevich, A.E. Berman, M.I. Bilan, Glycobiology 17 (2007) 541–552.
Teruya, T. Konishi, S. Uechi, H. Tamaki, M. Tako, International Journal of Biological Macromolecules 41 (2007) 221–226.
S. Vishchuk, S.P. Ermakova, T.N. Zvyagintseva, Carbohydrate Research 346 (2011) 2769–2776.
Norbury, P. Nurse, Annual Review of Biochemistry 61 (1992) 441–468.
Nagamine, K. Hayakawa, T. Kusakabe, H. Takada, K. Nakazato, E. Hisanaga,
Iha, Nutrition and Cancer 61 (2009) 340–347.
Fukahori, H. Yano, J. Akiba, S. Ogasawara, S. Momosaki, S. Sanada, K. Kuratomi, Y. Ishizaki, F. Moriya, M. Yagi, Molecular Medicine Reports 1 (2008) 537–542.
A. Lockshin, Z. Zakeri, Journal of Cellular and Molecular Medicine 11 (2007) 1214–1224.
W. Johnstone, A.A. Ruefli, S.W. Lowe, Cell 108 (2002) 153–164.
Duprez, E. Wirawan, T.V. Berghe, P. Vandenabeele, Microbes and Infection 11 (2009) 1050–1062.
R. Sprick, H. Walczak, Biochimica et Biophysica Acta 1644 (2004) 125–132.
Ashkenazi, V.M. Dixit, Science 281 (1998) 1305–1308. [82] J. Li, J. Yuan, Oncogene 27 (2008) 6194–6206.
Eskes, S. Desagher, B. Antonsson, J.C. Martinou, Molecular and Cellular Biology 20 (2000) 929–935.
Desagher, A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, J.C. Martinou, Journal of Cell Biology 144 (1999) 891–901.
[85] M.O. Hengartner, Nature 407 (2000) 770–776.
Yamasaki-Miyamoto, M. Yamasaki, H. Tachibana, K. Yamada, Journal of Agricultural and Food Chemistry 57 (2009) 8677–8682.
Liang, C. Yan, N.F. Schor, Oncogene 20 (2001) 6570–6578.
H. Kaufmann, S. Desnoyers, Y. Ottaviano, N.E. Davidson, G.G. Poirier, Cancer Research 53 (1993) 3976–3985.
Rodriguez, Y. Lazebnik, Science Signaling 13 (1999) 3179–3184.
Kim, S. Park, J.Y. Lee, J. Park, BMC Gastroenterology 10 (2010) 1–11.
O. Jin, M.G. Song, Y.N. Kim, J.I. Park, J.Y. Kwak, Molecular Carcinogenesis 49 (2010) 771–782.
Martin, C. Reutelingsperger, A.J. McGahon, J.A. Rader, R. Van Schie, D.M. LaFace, D.R. Green, Journal of Experimental Medicine 182 (1995) 1545–1556.
H. Hyun, S.C. Kim, J.I. Kang, M.K. Kim, H.J. Boo, J.M. Kwon, Y.S. Koh, J.W. Hyun,
D.B. Park, E.S. Yoo, Biological & Pharmaceutical Bulletin 32 (2009) 1760–1764.
[94] L. Chang, M. Karin, Nature 410 (2001) 37–40.
Wada, J.M. Penninger, Oncogene 23 (2004) 2838–2849.
S. Smalley, International Journal of Cancer 104 (2003) 527–532.
Whitmarsh, R. Davis, Oncogene 26 (2007) 3172–3184.
Suthiphongchai, P. Promyart, S. Virochrut, R. Tohtong, P. Wilairat, Oncology Research 13 (2003) 253–259.
Liao, L. Wang, X. Zhang, M. Liu, A. Ai zheng, Chinese Journal of Cancer 25 (2006) 1238–1245.
Lee, J.S. Kim, E. Kim, PLoS ONE 7 (2012) e50624.
S. Wu, J.R. Wu, C.T. Hu, Cancer and Metastasis Reviews 27 (2008) 303–314.
Busch, S.J. Renaud, E. Schleussner, C.H. Graham, U.R. Markert, Experimental Cell Research 315 (2009) 1724–1733.
Y. Lee, S.P. Ermakova, T.N. Zvyagintseva, K.W. Kang, Z. Dong, H.S. Choi, Food and Chemical Toxicology 46 (2008) 1793–1800.
Shaulian, M. Karin, Nature Cell Biology 4 (2002) 131–136.
C. Hsu, M.R. Young, J. Cmarik, N.H. Colburn, Free Radical Biology & Medicine 28 (2000) 1338–1348.
C. Lake, R. Vassy, M. Di Benedetto, D. Lavigne, C. Le Visage, G.Y. Perret, D. Letourneur, Journal of Biological Chemistry 281 (2006) 37844–37852.
Rouzet, L. Bachelet-Violette, J.M. Alsac, M. Suzuki, A. Meulemans, L. Louedec,
Petiet, M. Jandrot-Perrus, F. Chaubet, J.-B. Michel, Journal of Nuclear Medicine 52 (2011) 1433–1440.
Bachelet, I. Bertholon, D. Lavigne, R. Vassy, M. Jandrot-Perrus, F. Chaubet,
Letourneur, Biochimica et Biophysica Acta 1790 (2009) 141–146.
Folkman, Nature Medicine 1 (1995) 27–30.
Klagsbrun, P.A. D‘Amore, Annual Review of Physiology 53 (1991) 217–239.
J. Nelson, Journal of the National Cancer Institute 90 (1998) 960–963.
Koyanagi, N. Tanigawa, H. Nakagawa, S. Soeda, H. Shimeno, Biochemical Pharmacology 65 (2003) 173–179.
Arya, H. Ahmed, N. Silhi, M. Williamson, H.R. Patel, Tumor Biology 28 (2007) 123–131.
Kryczek, S. Wei, E. Keller, R. Liu, W. Zou, American Journal of Physiology Cell Physiology 292 (2007) 987–995.
Lv, Q. Song, Q. Shao, W. Gao, H. Mao, H. Lou, X. Qu, X. Li, Journal of Pharmacy and Pharmacology 64 (2012) 604–609.
Liu, J. Wang, A.K. Chang, B. Liu, L. Yang, Q. Li, P. Wang, X. Zou, Phytomedicine 19 (2012) 797–803.
Y. Hsu, T.Y. Lin, P.A. Hwang, R.H. Chen, S.M. Tsao, J. Hsu, Carcinogenesis 34 (2013) 874–884.
Company: SeaHerb Co., LTD.
CEO: Oh, Chungheon
Address: 276, Solchi-ro, Baebang-eup, Asan-si, Chungcheongnam-do, S.Korea
CS Center: mekabu@naver.com
Tel: +82 41-549-6466
Email: sh@seaherb.com
www.seaherb.com